Proc. of Second World Avocado Congress 1992 pp. 505-514
Origin of and Taxonomic Relationships within the Genus Persea
Rainer W. Scora and Bob O. Bergh
Department of Botany and Plant Sciences, University of
California, Riverside, CA 92521, USA
Abstract. The genus Persea in the Lauraceae belongs to the oldest subtribe of this family. From its origin in the western African Gondwanaland flora, its ancestral species migrated to Asia, and via Europe to North America, and via Africa to South America, probably by Paleocene time. When North and South America joined in the late Neogene, the genus was united again. Mountain building in Central America created new habitats in which speciation could take place. It was there that Persea americana may have evolved from tertiary and quaternary progenitors. Several subspecies are recognized, namely americana, guatemalensis, drymifolia, nubigena, steyermarkii and floccosa. The first two have an essential leaf oil profile which is essentially similar, characterized by the sesquiterpene caryophyllene. A very similar profile is that of Aguacate de Mico, recently described as P. tolimanensis. The profiles of Guaslipe (P. primatogena) are also similar to this Guatemalan complex. The profiles of ssp. nubigena and steyermarkii fit the projected ancestry of ssp. guatemalensis. With ssp. tolimanensis, they may be offshoots of an extinct ancestor.
Ssp. drymifolia from Mexico is distinguished by the acquisition of a larger quantity of the monoterpene estragole, which reaches 60% of the essential oil and can be used as taxon identification and for hybrid determination. Aguacate de Anis with an additional huge amount of the monoterpene anethole should be viewed as a natural variation of ssp. drymifolia, or chemically as a chemical race. Persea schiedeana remains the other species of subgenus Persea. Material to investigate Aguacate de Montana (P. zentmyerii) and Aguacate de Cimarron, Aguacatillo (P. paryifolia), was not available for study. Persea indica, a naturally surviving Gondwanaland species, also present in the Laurasian Neogene, differs from the other tested species of subgenus Eriodaphne by its quantitatively extremely low monoterpene profile. Persea pachypoda had a unique monoterpene camphor peak. None of the Asian species of Persea has been available for study.
The genus Persea (Clus.) Miller belongs to the family Lauraceae. This family has arisen from woody magnolian forebears and represents a terminal group which is specialized by reduction and fusion and from which no extant plant taxa have since evolved. With the Annonaceae, Magnoliaceae, and Proteaceae, it ranks among the oldest recorded flowering plants.
The family Lauraceae is economically important. It has been used by mankind for spices like cinnamon from Cinnamomum zeylanicum Nees, cassia from C. cassia Blume, and sassafras from Sassafras albidum (Nutt.) Nees; camphor for medicinal and industrial uses from C. camphora L.; drugs from Aniba coto (Rusby) Kosterm.; bases for perfume oils from C. cassia, Aniba duckei Kosterm., and A. rosaeodora Ducke; seedfat from Litsea sebifera Pers. and Laurus nobilis L.; demera greenheart from Ocotea rodiaei (Schomb.) Mez; and other timber from Persea indica (L.) Spreng., P. nanmu Oliv. Mezilaurus itauba (Meissn.) Taub., Ocotea bullata (Burch.), E. May, and Eusideroxylon zwageri T. et B.; edible fruit from Persea americana Miller and P. schiedeana Nees.; such ornamentals as Persea indica L.
Kostermans in his worldwide review of the Lauraceae (1957) has subdivided the family into 2 subfamilies, namely Lauroideae and Cassythoideae. The former he divided into 5 tribes and 8 subtribes, namely tribe Perseeae into subtribes Perseineae and Beilschmiediineae; tribe Cinnamomeae into Cinnamomineae and Anibineae; tribe Litseeae into Litseineae and Lauriineae; tribe Cryptocaryeae into Cryptocaryineae and Eusideroxylineae; and into tribe Hypodaphneae. We view the Perseineae with the Beilschmiediineae (frequent companions in the fossil record) as very old, a concept shared by Gottlieb (1972), who based his study on the presence of alkaloid, arylpropanoid, flavanoid and terpenoid constituents. The family is very old indeed. Persea was part of the African Gondwanaland flora in paleocene times; from there its taxa migrated to Asia, where some 80 species exist today. Other species migrated to southwestern Europe, and from there to North America when Eurasia and North America were in direct contact into the late Cretaceous (Tarling, 1971) and islands were scattered along the mid-Atlantic ridge into Paleogene times (Raven, 1973). Other species, many from subgenus Eriodaphne, migrated from Africa via Antarctica, which at that time and with its geographic location close to Africa, was covered with tropical rainforests common to South America. The many islands existing along the Mid-Atlantic Ridge and its flanks were an alternate route which afforded ready migration from Africa to South America across the opening Atlantic into the early Tertiary.
Tropical Africa's flora was subsequently impoverished by the spread of dry climate beginning near the close of the Oligocene (Axelrod, 1972) and by the development of the Benguela Current, when glaciation was initiated on Antarctica in the early Miocene (Hayes et al., 1973), as well as by several periods of ice age aridity during the Quaternary.
Persea fossil distribution is different from its present range. It grew in regions which now are temperate and even polar. Persea is known in North America from at least Eocene time onward (MacGinitie, 1941). Nevertheless, its fossil finds in California (Schroeder, 1968), Nevada (Axelrod, 1956), France (Boulay, 1922), and Spain (Almera, 1907) suggest a former Neotropical-Africa European-Southeast Asian paleo-distribution for this plexus. Its present disjunct distribution in the Neotropics and in southeast Asia is due to extinction in Europe and Africa, with only P. indica (L.) Spreng. surviving as endemic in the laurel forests of the north coast of the Canary Islands, which with Madagascar are the two principal refuges for the remnants of the formerly far more widespread African laurel forest taxa (Raven and Axelrod, 1974).
In South America, the genus Persea is found chiefly in the montane forests of east central to southern Brazil. Here, half of the species of section Aurataea Kopp are native. Most remaining South American species populate the upper rainforests on the slopes of the Andes. All species of section Mutisaea Kopp are found here except one, and all of section Hexanthera Mez except three. Also, two endemics are present in the Guayana Highlands (Kopp, 1966), and P. linque (Nees) reaches into the temperate zones of Chile. From the mountain chains of Central America, the genus continues along the Sierra Madre Occidental to the Sierra Madre Oriental of Mexico. Only P. caerulea (Ruiz and Pavon) Mez occurs in both South and Central America. The tropical flora of South America which had evolved in isolation from an original African stock had progressively established itself in areas of appropriate climate in Central and North America, including the West Indies, by Paleogene time. In the Pliocene, when the isthmus of Tehuantepec and the Guyana Shield were linked by volcanic and other islands, a mixing of biota took place. These taxa were followed by others in larger numbers in late Neogene, after the establishment of the Panamanian land connection about 5.7 millions years ago. The indigenous West Indian mountain species, Persea urbaniana Mez, seemingly originated in northern South America, and P. alpigena (Swartz) Spreng. probably in Central America. Persea borbonia (L.) Spreng. and P. palustris (Raf.) Sarg. reached the southeastern coastal plain of the United States; their affinity lies with the West Indian species (Kopp, 1966).
Materials and Methods
For essential oil analysis, 500 g of healthy leaves were randomly harvested from seedling trees of the Persea Species Collection at the University of California South Coast Field Station in Irvine, CA. Leaf material of low-land taxa (West Indian) was kindly provided by the USDA-Miami station. The leaves were macerated and steam distilled in a Clevenger apparatus for 2 h. The resulting oils were kept under nitrogen until injected into a Shimadzu Mini-3 G.C. with F1D detector and 50:1 split ratio. A G&W DB-5 fused silica capillary column was used with 0.25 mm i.d. and 60 m length and 0.25 µm coating thickness. The injector temperature was 200C. The oven temperature programmed from 65C initial temperature, 30 min initial time, increase of 1o/min to 200C and 25 min final time.
N-octane and n-eicosane were added to some samples for standardization of retention times. For mass spectra identification, a Hewlett-Packard 5890II G.C., a 5971A mass selective detector, and G1030A MS computer station were used (Figs. 9, 10). A capillary direct interphase heated at 280C delivered column output into quadrapole MS spectrophotometer with electrons of 70 eV energy for electron impact ionization. One µL of appropriately diluted oil in hexane was injected in splotless mode. Injector temperature was 270C and helium flow 22 cm/sec. All other parameters were the same as for the Shimadzu. A few mass spectra were identified by the NIST/EPA/MSDC data base (49000 spectra) stored in the computer station. All others were identified by comparison with data provided in Adams (1989).
Results and Discussion
The genus Persea is subdivided into two closely related subgenera, namely Persea, to which the edible avocados belong, and subgenus Eriodaphne Nees. The essential leaf oil patterns show clearly the division into mono- and sesquiterpenes. Their order of elution was: n-hexanol, hexenol, 2-hexenal, dimethyl benzene, n-nonane, tricyclene, α-pinene, camphene, ß-pinene, ß-myrcene, decane, 4-carene, α-terpinene, p-cymene, d-limonene, ß-phellandrene, 1,8-cineole, cis-ocimene, trans-ocimene, gamma-terpinene, terpinolene, linalool, benzene methanol, camphor, terpinen-4-ol, α-terpineol, estragol, anethol, undecanone, delta-elemene, α-cubebene, ß-patchoulene, ß-bourbonene, delta-elemene, methyl eugenol, bergamotene, ß-caryophyllene, ß-gurjunene, α-cadinene, α-gurjunene, α-carophyllene, gamma-muurolene, germacrene D, valencene, α-muurolene, α-farnesene, gamma-cadinene, delta-cadinene, ß-calacorene, 3-methoxycinnamaldehyde, copaene, caryophyllene oxide, cubenol, tau-cardinol, α-cadinol, Patchouli alcohol, cis-ß-santalol.
The type species P. americana Mill, consists of three taxa, sometimes termed horticultural races (Mexican, Guatemalan, and West Indian), later botanically considered varieties. Taxonomic usage today usually treats easily recognizable differences within a species as subspecies. More subtle differences are considered to be part of the natural variations within a species population. We have named the West Indian subspecies the Lowland taxon, since today's consensus agrees that this group evolved on the west coast of Central America and the new name would reflect both the western and eastern sides of the mountain range.
The leaf oil patterns of ssp. guatemalensis (Fig. 1) possess the usual array of monoterpenes and sesquiterpenes, dominated by caryophyllene, with a few esters at the end. Ssp. americana is similar, but almost lacking the sesquiterpenes esters and having small amounts of cisocimene, when compared with guatemalensis. Ssp. drymifolia (Fig. 2) is dominated by the monoterpene estragole (Fig. 3), reaching up to 1% of fresh leaf weight. This component can be used as a taxonomic character (King and Knight, 1987), indicating - in hybrids involving a Mexican parent -the approximate contribution of the Mexican gene pool.* These findings are in contrast to the concept of Williams (1977), who placed the Guatemalan in a species separate from the Mexican and West Indian types, and who considered the West Indian taxon a selection from the Mexican. Aguacate de Anis (Fig. 2) is of the Mexican type, with an additional accumulation of anethol (Fig. 4), which differs from estragole only in the position of its double bond. Taking the total biological make-up into consideration, we consider this taxon a chemical variant of the Mexican drymifolia.
It might be interesting to speculate as to whether P. americana is mono- or polyphyletic. We consider the total evidence inadequate for any such determination. Garcia and Tsunewaki (1977) postulated on the basis of peroxidase isozyme relative band number that the Puebla-Veracruz region was the center of origin of the Mexican race, and from relative strain frequency within each taxon, they believed that the Guatemalan type is the most ancient form. We do not find any of their evidence persuasive.
Also probably meriting subspecies standing is P. floccosa Mez. (Fig. 5), which has a rather Guatemalan-type pattern. So does a plant designated P. primatogena (guaslipe) Williams and Molina (Fig. 6) in UCR's Persea collection; the latter species may not, however, belong in the genus Persea (Zentmyer, personal communication). Aguacate de Mico or P. tolimanensis (Schieber and Zentmyer) (Fig. 6) also exhibits a Guatemalan-type pattern, with the addition of terpinen-4-ol. P. steyermarkii Allen also is similar to the guatemalan type, with additional sesquiterpene esters. Schieber and Zentmyer (1977) advanced the concept that P. steyermarkii and nubigena Williams were ancestors of the guatemalan criollos. This was supported by DNA analysis by Furnier et al. (1990). In 1987, Schieber and Zentmyer widened this concept to include additionally, P. zentmyerii Schieber and Bergh and P. tolimanensis. There is nothing in our data that would contradict this concept. However, the above four taxa, instead of being the direct guatemalensis ancestors, may rather be offshoots of still earlier antecedents which have not yet been identified, or which have died out.
Persea borbonia, in subgenus Eriodaphne, is characterized by cis-ocimene. This species is Phytophthora-resistant, as is P. caerulea (Fig. 7), which is governed by ß- and α-caryophyllene. Kopp (1966) thought P. caerulea to be conspecific with P. skutchii Allen (Fig. 8); however, in addition to quantitative differences of individual terpene components, there are also qualitative differences of terpinen-4-ol and sesquiterpene esters.
P. pachypoda Nees is the only species in the genus in which we detected camphor (Fig. 3). This bicyclic monoterpene elutes early, at 55 minutes, despite the fact that it is one of the few essential oils which are solid at room temperature. Its biosynthetic route from α-pinene may lead to camphor or borneol. We do not expect any further chemical variation in this species.
Our results demonstrate that chemical analyses such as essential oil comparisons provide useful data for helping to determine avocado relationships, for example on the specific level in P. pachypoda, on the subspecific level in ssp. drymifolia, and on the chemical varietal level in Aguacate de Anis.
The
volatiles designated in this manuscript as sesquiterpene esters have now been
identified as avocadofurans by P. Weyerstahl (Berlin).
*Bergh et al., (1973) reported on the
estragole content of P. americana and listed 79% for 'Waldin', a low-land or
West Indian taxon. This was obviously a mistake. The records for this
particular tree from which the leaf material for the chemical analysis was
taken showed that this tree, grafted on Mexican rootstock, was planted in 1956
at UCR. Seemingly, the scion had died and the rootstock which had taken over
provided the leaf material for the chemical analysis. The first fruit from this
tree were harvested two years later and were small and dark-skinned, further
confirming the Mexican heritage.
Support for
purchase of the mass selective detector used in this study was provided by a
grant from the National Science Foundation, DIR-8914574.
Literature Cited
Adams. R.P. 1989. Identification of essential oils by ion trap mass spectroscopy. Academic Press, Inc. San Diego, New York, Berkeley, pp. 302.
Almera,
J.J. 1907. Flora pliocenica de los abrede doves de Barcelona. Mem. Rest. Acad.
Ciencias Artes Barcelona 3:321-335.
Axelrod, D.I. 1956. Mio-Pliocene floras from
west-central Nevada. Univ. Calif. Pub. Geol. Sci. 33:1-316.
Axelrod, D.I. 1972. Ocean-floor spreading in relation to ecosystematic problems. Univ. Arkansas Mus. Occas. Paper 4:15-76.
Bergh,
B.O., R.W. Scora, and W.B. Storey. 1973. A comparison of leaf terpenes in Persea
subgenus Persea. Bot. Gaz. 134:130-134.
Boulay, N. 1922. La flore pliocene de la Vallie du Rhone.
Ann.
Sci. Natur. Bot. Ser. 10:73-366.
Fournier, G.R., M.P. Cummings, and M.T. Clegg. 1990. Evolution of the avocados as revealed by DNA restriction fragment variation. J. Heredity 81:183-188.
Garcia, A., and K. Tsunewaki. 1977. Cytogenetical studies in the genus Persea (Lauraceae). III. Electrophoretical studies on peroxidase isozymes. The Jap. J. Genetics 52:379-386.
Gottlieb, O.R. 1972. Chemosystematics of the Lauraceae. Phytochemistry 11:1537-1570.
Hayes, D.E., L.A. Frakes, P. Barrett, D.A. Burns, Pei-Hsin Chen, A.B. Ford, A.G. Kaneps, E.M. Kemp, D.W. McCollum, D.J.W. Piper, R.E. Wall, and P.M. Webb. 1973. Leg 28 deep-sea drilling in the southern ocean. Geotimes 18:19-24.
King, J.R. and R.J. Knight. 1987. Occurrence and assay of estragole in the leaves of various avocado cultivars. J. Agric. Food Chem. 35:842-844.
Kopp, L.E. 1966. A taxonomic revision of the genus Persea in the western hemisphere (Persea-Lauraceae). Mem. NY Bot Card. 14:1-120.
Kostermans, A. J. G.H. 1957. Lauraceae Reinwardtia 4:193-256.
MacGinitie, H.D. 1941. A middle Eocene flora from the central Sierra Nevada. Carnegie Inst. Wash. Publ. 534:1-178.
Raven, P.M. 1973. The evolution of Mediterranean floras, p. 215-217. In: F. Castri and H. A. Mooney (eds.). Mediterranean Type Ecosystems. Springer-Verlag, New York
Raven, P.M. and D.I. Axelrod. 1974. Angiosperm biogeography and past continental movements. Annals Missouri Bot. Garden 62:539-674.
Schieber, E. and G.A. Zentmyer. 1977. Exploring for Persea in Latin America. Proc. 1st Inter. Trop. Fruit Short Course. The Avocado. Gantz et al., ed., Univ. Florida, Gainesville: 16-20.
Schieber, E. and G.A. Zentmyer. 1987. Exploring for sources of resistance among Persea americana var. guatemalensis and Persea schiedeana in Middle America. S. A. Avocado Growers' Assn. Yrbk. 10:20-21.
Schroeder, C.A. 1968. Prehistoric avocados in California. Calif. Avocado Soc. Yrbk. 1968:29-34.
Schroeder, C.A. 1969. Fossil avocado leaf. Calif. Avocado Soc. Yrbk. 1969:59-60.
Tarling, D.H. 1971. Gondwanaland paleomagnetism and continental drift. Nature 229:17-21.
Williams, L.O. 1977. The avocados, a synopsis of the genus Persea, subg. Persea. Economic Botany 31:315-320.
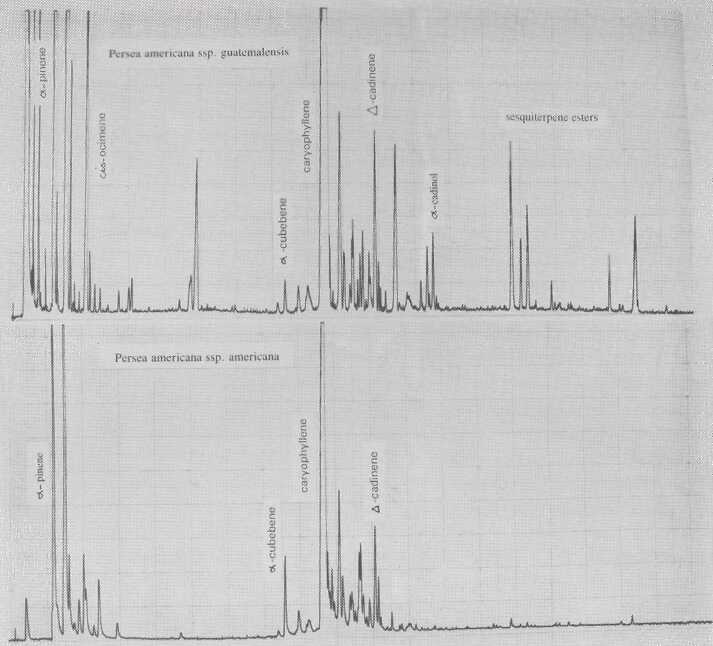
Fig. 1. Gas chromatograms of Persea americana ssp. guatemalensis and ssp. americana.
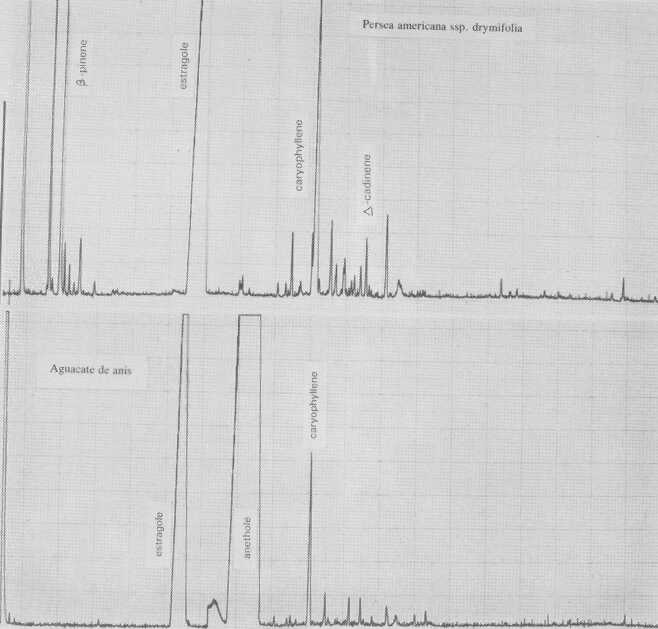
Fig. 2. Gas chromatograms of Persea americana ssp. drymifolia and of "Aguacate de anis".
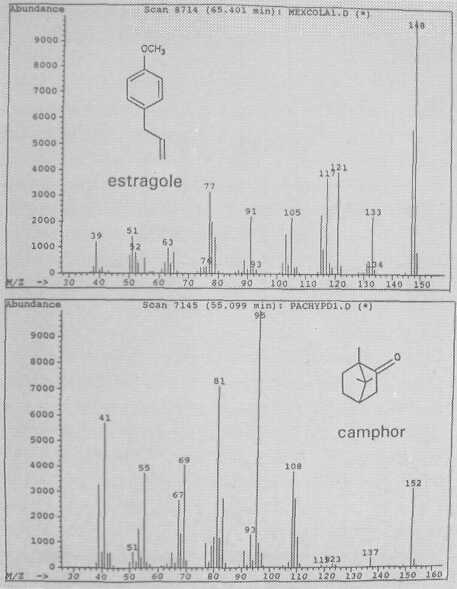
Fig. 3. Mass spectra of monoterpenes estragole and camphor.
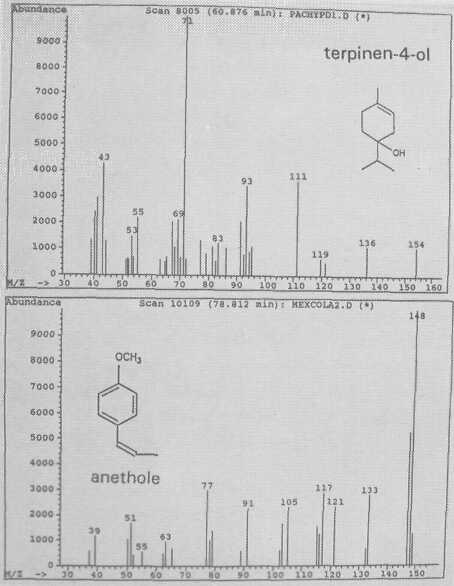
Fig. 4. Mass spectra of monoterpenes terpinen-4-ol and of anethole.
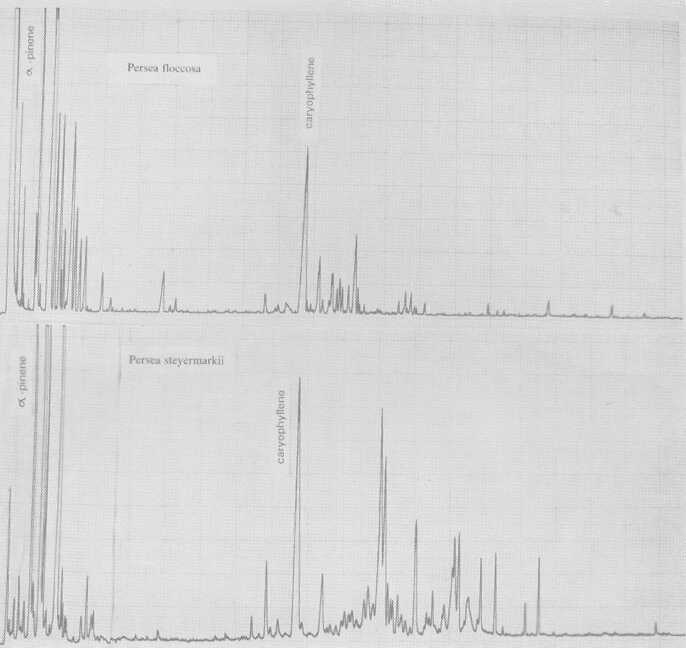
Fig. 5. Gas chromatograms of Persea floccosa and of P. steyermarkii.
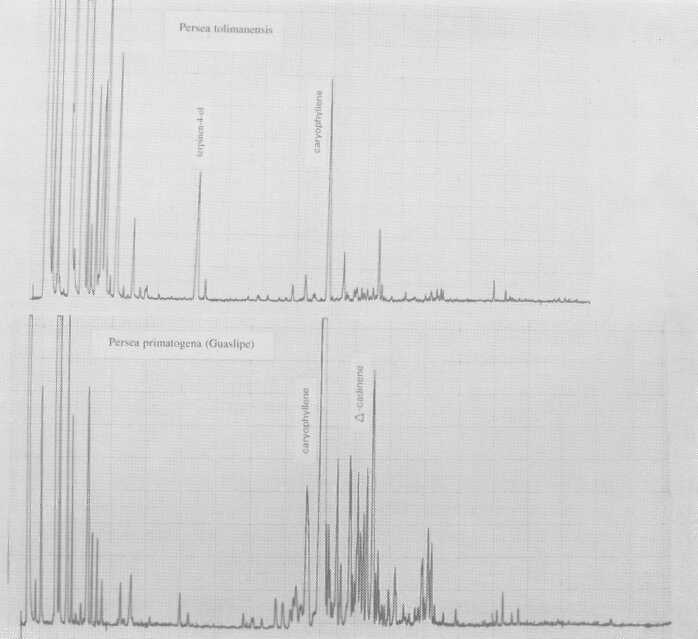
Fig. 6. Gas chromatograms of Persea tolimanensis and of P. primatogena (Guaslipe).
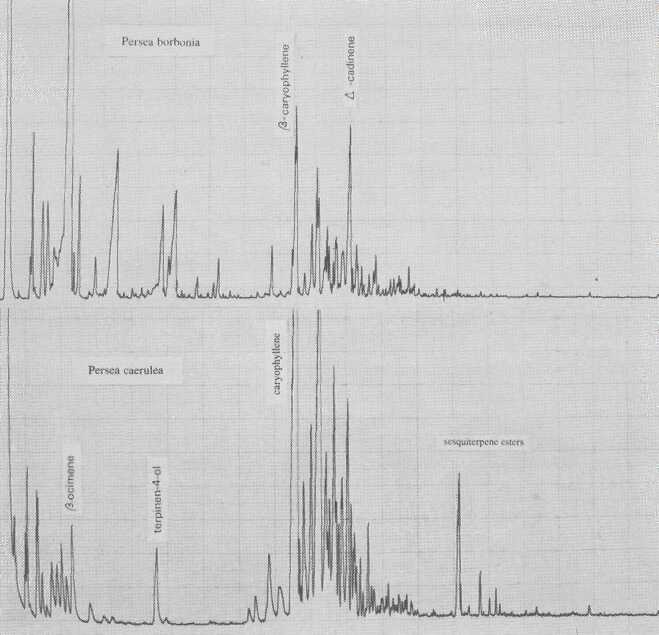
Fig. 7. Gas chromatograms of Persea borbonia and of P. caerulea.
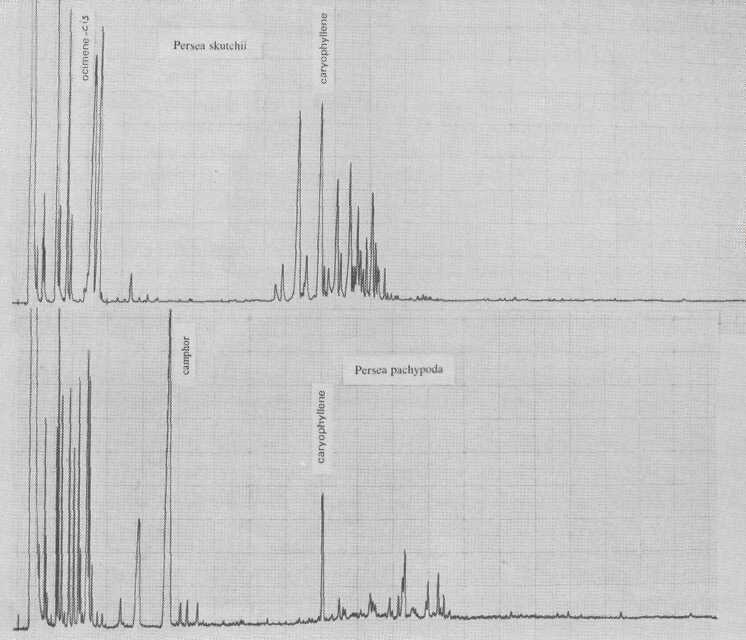
Fig. 8. Gas chromatograms of Persea skutchii and of P. pachypoda.
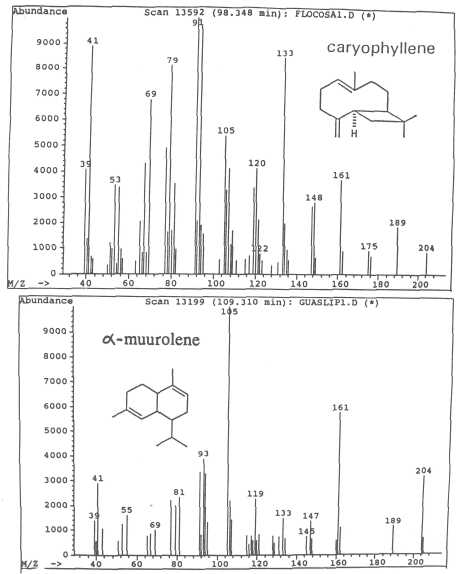
Fig. 9. Mass spectra of sesquiterpenes caryophyllene and of α-muurolene.
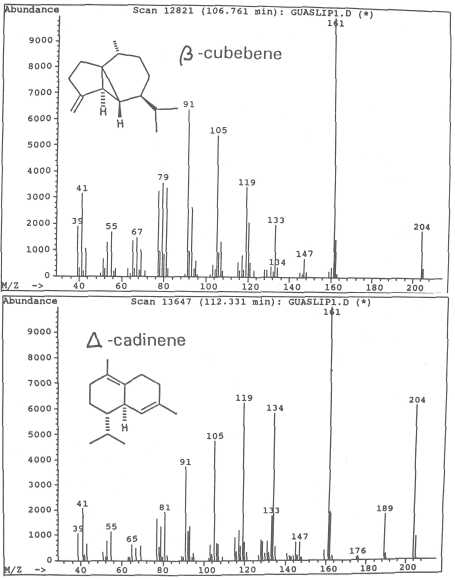
Fig. 10. Mass spectra of sesquiterpenes ß-cubebene and of ⌂-cadinene.