Proc. of
Regulation of Natural
Resistance of Avocado Fruit for the Control of
Department of Fruit and Vegetable Storage, ARO, The
Richard Plumbley
Natural Resources Institute,
Noel T. Keen and Jim J. Sims
Department of Plant Pathology,
Abstract. Avocado
fruit are free of Colletotrichum gloeosporioides symptoms
at harvest but can develop decay lesions during ripening and softening. The
resistance of unripe avocado fruit peel to fungal development was found to
result from the presence of the antifungal compound
1-acetoxy-2-hydroxy-4-oxo-heneicosa-12,15-diene. The
concentration of the compound in avocado peel decreased during ripening,
concomitantly with resumed development of previously quiescent infections. The
antifungal diene in ripening fruit is metabolized by
the enzyme lipoxygenase. The activity of this enzyme
is regulated by the presence of the flavan-3-ol, epicatechin.
Decrease in concentration of the endogenous inhibitor epicatechin
occurred during fruit ripening. Fruit resistance might be regulated in three
ways: 1) development of cultivars with a
naturally high concentration of epicatechin; 2) the use of antioxidants that
inhibit lipoxygenase; and 3) the induction of the antifungal diene
by high concentrations of CO2.
Colletotrichum gloeosporioides attacks avocado fruit during the growing season of the fruit. Conidia of the fungus germinate on the peel of the fruit and produce appressoria that germinate and penetrate the fruit cuticle. The fungus does, however, not develop further until the fruit are harvested and ripen. Only at this stage are symptoms of disease expressed. The period between infection of the fruit and disease development is known in fruit pathogen interactions as the quiescent stage. This period between infection and symptom development is actually a stage where fruit are resistant to pathogen attack.
The control of fungal decay in fruit was done in the past and present by synthetic fungicides that prevented the development of infections which occurred in the field or after harvest. We describe a new alternative way to prevent fruit decay, by modulating the natural resistance of fruit.
Several questions should be answered to develop this method of decay control. The first question is what is the mechanism of resistance of the particular fruit in which we are interested?
Materials
and Methods
Avocado fruit (cv. Fuerte) were used in all experiments. A single-spore isolate of C. gloeosporioides from decayed fruit was used in the experiments. Fruits were inoculated with a 10 μL drop of a spore suspension (106 spores/mL) placed at four different positions along both sides of the longitudinal axis of each of 20 harvested fruit. Fruit were observed daily for disease symptoms using a binocular microscope.
The antifungal diene was extracted from avocado peel and quantified as described by Prusky et al. (1982). The lipoxygenase (LOX) inhibitor, epicatechin, was extracted in 5 mM sodium phosphate buffer (pH 7.2), partially purified by flash chromatography, and quantified by HPLC as described by Prusky et al. (1985).
Results
and Discussion
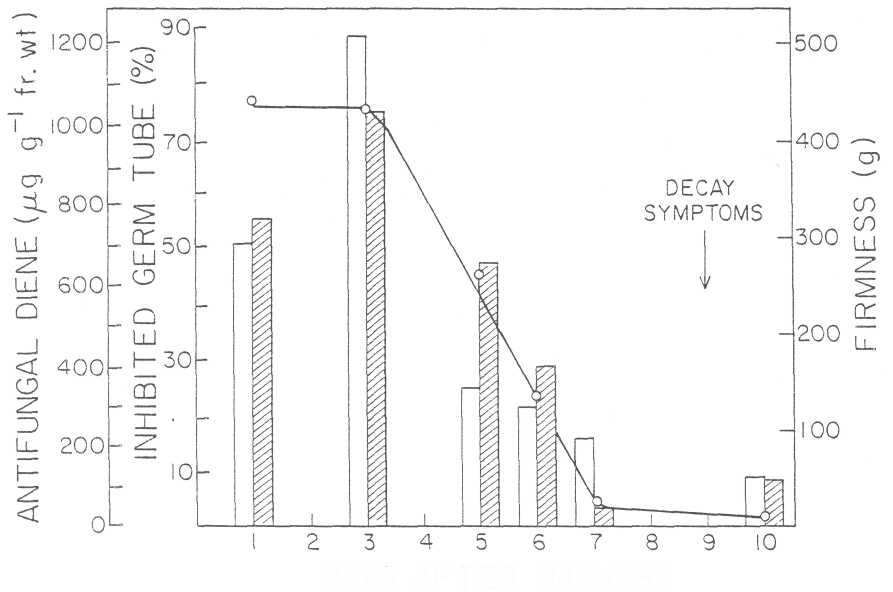
Our work revealed the presence of a preformed antifungal compound in the peel and flesh of unripe fruit, which was identified as 1-acetoxy-2-hydroxy-4-oxo-heneicosa-1 2,1 5-diene (Fig. 1). The effective dose for 50% inhibition of germ tube elongation was 450 μg/mL. The concentration of the antifungal diene in unripe fruit 1 to 3 days after harvest varied between 700 and 1230 //g/g FW of avocado peel and this represents an approximate concentration of 1600 μg/mL which is much higher than the ED50 of 450 μg/mL (Fig. 2). Five days after harvest the concentration decreased to 350 μg (equal to approximately the ED50) and after an additional 5 days, it was 124 μg/g FW. This represents an approximate concentration of 160 μg/mL, lower than the ED50 of this compound. At this stage symptoms are evident. The antifungal activity of this compound in similar extracts shown by the striated bars, decreases from 55% to 6% inhibition of hyphal elongation during the same period, indicating a close correlation between the concentration of the antifungal compound and the inhibition of activity of the crude extract.
Fruit susceptibility is therefore dependent on the metabolism and decrease in concentration of the antifungal diene. In the search for the possible enzymatic systems that might metabolize the antifungal diene, we found that avocado lipoxygenase might be involved with this decrease. Several experiments led us to this conclusion. First, we found that partially purified avocado lipoxygenase was able to oxidize the antifungal compound in vitro. Second, the activity of this enzyme in avocado fruit extracts increased during fruit ripening by 80% before symptoms of disease were observed (Fig. 3). Third, we were able to inhibit the enzyme by a specific inhibitor of lipoxygenase and at the same time delay decay development and the decrease of the antifungal diene. All this suggests avocado lipoxygenase as a possible enzyme involved in the diene breakdown.
Our interest was now directed to the regulation of lipoxygenase activity since this activation might result in the transformation of the resistance to the susceptible conditions of the fruit. A natural inhibitor of avocado LOX was isolated and identified as epicatechin. The flavan-3-ol epicatechin is known to be an antioxidant by its action as a free radical trap which results in its own oxidation. The inhibition of LOX by avocado peel extract decreased from 55% to 6% in ripe fruit when symptoms were observed. The actual concentration of epicatechin in this case was about 500 μg/g FW and decreased to about 8 to 30 μg/g FW before symptoms of Colletotrichum gloeosporioides infection developed.
In summary, the present results suggest that the transformation of a quiescent infection into an active infection or, in other words, of a resistant stage to a susceptible stage, is the result of the decrease of the antifungal diene, which is catalyzed by lipoxygenase activity and regulated by the decline of its inhibitor epicatechin (Fig. 4.).
The understanding of the system involved in fruit susceptibility led us to try to understand the possible regulation of resistance. Several different factors that might prevent the metabolism of the antifungal diene or either induce this particular process and lead to an increase in diene production resulting in both cases delay of disease development.
One of the possibilities is the use of postharvest treatments that inhibited lipoxygenase activity and consequently delayed the activation of the quiescent infection and decay development. Several antioxidants were tested (Prusky, 1988). The best results were obtained by the dipping of freshly harvested fruit in a mixture of butylated toluene and ascorbic acid.
These treatments were equally good for the control of stem end rot and as good as treatment with the commercial fungicide, prochloraz (Fig. 5). Currently there is a commercial formulation of the antioxidant butylated hydroxyanisole (BHA) known as Xedaphen-20 that contains 20% BHA that significantly reduces the incidence of postharvest decay in avocado.
Another possibility to control disease by modulation of natural resistance in susceptible cultivars is the induction of higher concentrations of the antifungal diene. It was observed that if we expose freshly harvested fruit to an atmosphere of 30% CO2 for 24 h, we were able to induce higher concentrations of the antifungal diene during ripening and the anthracnose development was delayed (Fig. 6).
The third possibility to prevent development of anthracnose is the selection of cultivars with high epicatechin concentrations in the peel (Prusky et al., 1988). It was found that cultivars more resistant to anthracnose such as Nabal showed higher initial amounts of epicatechin and a slower decrease which delayed the activation of quiescent infections.
Conclusions
We have elucidated what appears to be the mechanism of avocado fruit resistance to postharvest disease, described the antifungal diene as the main antifungal compound involved in the resistance and epicatechin as the regulator of the mechanism.
There is evidence which indicates that we may prevent decay development by the use of: 1) antioxidant treatments; 2) induction of resistance by CO2 treatment; and 3) selection of resistant cultivars with high concentrations of epicatechin.
We
acknowledge BARD for funding
this work.
Literature
Cited
Prusky, D. 1988. The use of antioxidants to delay the onset of anthracnose and stem end rot in avocado fruit after harvest. Plant Disease. 72:381-384.
Prusky, D.,
Prusky, D., N.T. Keen, J.J. Sims, and S.L. Midland 1982. Possible involvement of an antifungal compound in latency of Colletotrichum gloeosporioides in unripe avocado fruit. Phytopathology. 72:1578-1582.
Prusky, D.,
![]()
Fig. 1. The chemical structure of the antifungal diene.
DAYS AFTER HARVEST
Fig. 2. Fruit firmness (striated bar), antifungal activity (o-o), and concentration of the antifungal diene (open bar) in crude extracts from avocado cultivar Fuerte at different stages after harvest. The arrow denotes the first visible decay symptoms.
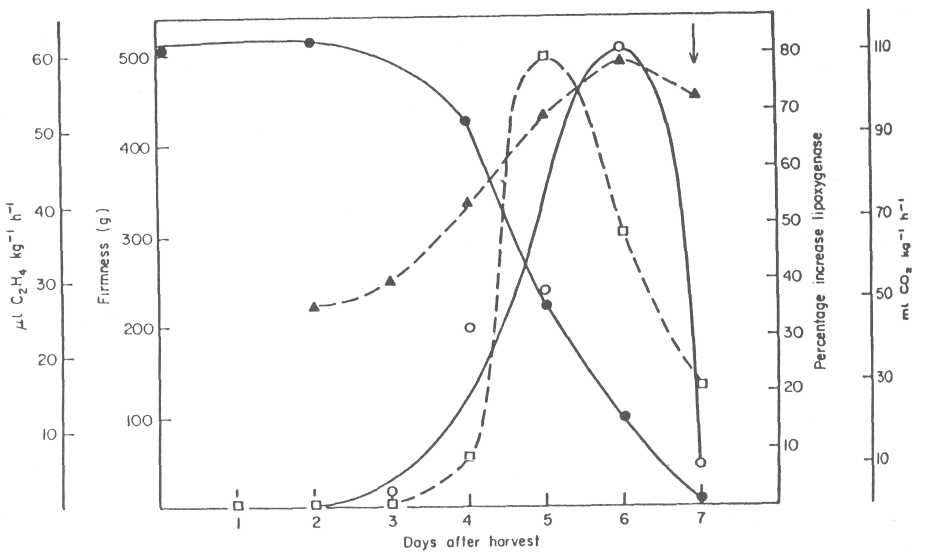
Fig. 3. Lipoxygenase specific activity (o-o), evolved ethylene (D-Q), respiration U--A) and fruit firmness (•--•) in 'Fuerte' avocado fruit incubated at 20C. Lipoxygenase activity represents the increase in specific activity of the enzyme in fruit extracted at different times after harvest in relation to freshly harvested fruit. Arrow indicates appearance of symptoms.
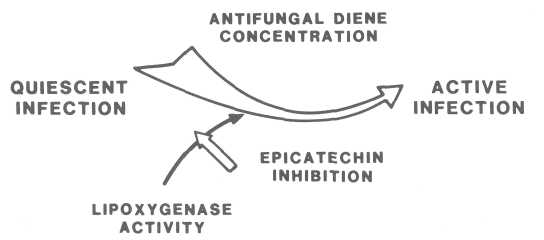
Fig. 4. Sequence of reactions leading to
active infections of Co/letotrichum gloeosporioides in ripening avocado fruit. Lipoxygenase-catalyzed decrease in diene
concentration facilitates activation of quiescent infection. Epicatechin inhibits lipoxygenase
activity.
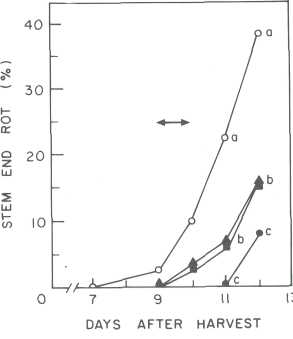
Fig. 5. Effect of postharvest
dips in antioxidants and fungicide on the natural incidence of Diplodia natalensis stem
end rot on stored avocado fruit (cv. Fuerte). Fruit were dipped in a mixture of 0.1 mM BHT and 0.5% ascorbic acid (•), 0.1 mM
BHT and 0.1% citric acid (•), or 900 μg/mL prochloraz U). Control
fruit (o) were dipped in water. Fruits were stored at 20C throughout the
experiment. After treatment, the fruit were point-inoculated with a spore
suspension of C. gloeosporioides (ca. 10
spores/mL). The fruit were incubated overnight at 25C
and then transferred to 20C until disease symptoms were visible.
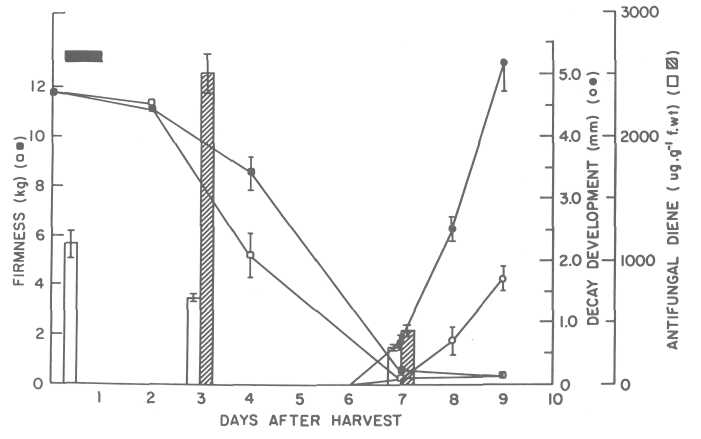
Fig. 6. Fruit firmness, diene concentration and decay development in avocado fruit (cv. Fuerte) treated with 30% C O2. Freshly harvested fruit were sealed in flow-through jars, treated under a stream of CO2in air (100 mL/mL) for 24 h and then transferred to normal atmospheric conditions: ٱ-o control fruit, ■-o CO2 treated. After treatment, the fruit were point-inoculated with a spore suspension of C. gloeosporioides (ca. 106 spores/mL). The fruit were incubated overnight at 25C and then transferred to 20C until disease symptoms were visible.