Proc. of
Ultrastructure of Avocados: Ripening, Chilling Injury, and Isolation of Idioblast Oil Cells
K. A. Platt-Aloia and W. W.
Thomson
Department of Botany and Plant Sciences,
Abstract. Ripening and chilling injury of avocado (Persea
The ripening of fruits is a complex process, including several different physiological and biochemical changes. These changes are reflected in structural modifications at the cellular level. Ripening of avocados involves, primarily, a softening of the fruit mesocarp. Biochemical analyses of avocados have shown large increases in the activities of wall hydrolytic enzymes during ripening (Awad and Young, 1979). Structural studies using the electron microscope correlated the dissolution of the cell walls with this increased enzyme activity and concomitant softening of the fruit (Platt-Aloia et al., 1980; Platt-Aloia and Thomson, 1981).
Mature avocados begin ripening when they are harvested. The process can be slowed considerably by low temperature storage (4-6C); however, extended cold storage results in chilling injury, which is manifested as discoloration of the mesocarp, improper softening, and off-flavor (Couey, 1982). A previous study on the ultra-structure of chilling-injured avocados reported apparent damage to the cell membrane, evident as phase separations of the phospholipids and proteins of the bilayer (Platt-Aloia and Thomson, 1987; Platt-Aloia, 1988).
The major storage component of the avocado fruit is oil, primarily triglycerides (Biale and Young, 1971). This oil is stored as droplets in the cytoplasm of the parenchyma cells of the mesocarp (Platt-Aloia and Thomson, 1981). Additionally, approximately 2% of the cells of the mesocarp are specialized (idioblast), oil cells (Cummings and Schroeder, 1942; Platt-Aloia et al., 1983). The oil contained in these specialized cells has a different appearance at both the light (Scott et al., 1963) and electron microscope (Platt-Aloia et al., 1983) levels. The composition and possible function of this oil, however, are unknown.
The objectives of this article are to review the structural changes that occur in the avocado fruit cells during ripening and with chilling injury, and to describe the effects of these processes on the ultrastructure of the specialized oil cells. We also report preliminary results on isolation of the idioblasts, and biochemical characterization of the oil they contain.
Materials and Methods
Avocados (Persea
Samples for electron microscopy were fixed in 1% glutaraldehyde in 50 mM cacodylate buffer, pH 7.0, at room temperature for 2 to 4 hours, rinsed in buffer, postfixed overnight in cacodylate-buffered 1 % OsO4, dehydrated in acetone, and embedded in Spurr's resin (Spurr, 1969). Samples for freeze fracture were not fixed. Tissue was frozen in liquid propane, and replicas were prepared in a Balzers freeze fracture apparatus according to the methods described by Platt-Aloia and Thomson (1982).
The idioblast oil cells were isolated from soft, ripe fruit. Tissue was homogenized in a Ten Broeck homogenizer with distilled water. The homogenate was filtered through a 200 µm nylon mesh to remove vascular strands. The filtrate was then filtered through a 48 µm nylon mesh. The residue was thoroughly washed with water and centrifuged at 80 x g for 2 to 3 min. The pellet contained the isolated oil cells, and some larger cell debris including cell walls and small pieces of vascular strands.
Lipids of the isolated oil cells were extracted by a modification of the technique of Bligh and Dyer (1959). Extraction was with chloroform:methanol (2:1), followed by centrifugation. The lower phase was washed twice with distilled water to remove water soluble impurities. The lower phase was then concentrated under N2 gas. Thin layer chromatography was performed on silica plates, using a solvent system of benzene:ether:acetic acid (80:20:1), and developed with iodine vapors.
Results
Parenchyma cells of mature, unripe avocado fruit contain large amounts of lipid, the normal complement of organelles, and intact cell walls (Figs. 1, 7). The endoplasmic reticulum (ER) of these cells is visible in thin sections as a network of sheets and tubules (Fig. 1). The cell walls are of moderate electron density, consisting of cellulose fibrils embedded in a matrix.
As ripening progresses, the ER swells and vesiculates (Fig. 2). The wall becomes more fibrous in appearance with apparent loss of the matrix (Fig. 2).
In very ripe, post-climacteric fruit, the cytoplasm and organelles retain essentially the same organization as in unripe fruit, except for the ER which is highly vesiculated, and the cell walls, which are almost electron transparent (Fig. 3).
Freeze fracture electron microscopy of the plasma membrane of both unripe, and ripe fruit appear very similar (Figs. 4, 5). In both cases, the membrane fracture indicates an intact bilayer with protein particles evenly distributed throughout. However, the cell membrane of fruit that had been stored at 6C until it exhibited chilling injury had undergone changes in organization. Figure 6 is a freeze fracture replica of the plasmalemma of a chilling-injured avocado fruit. The membrane components have become redistributed to form areas of lipid that are depleted of protein particles. This redistribution indicates a phase separation of the membrane lipids into gel and fluid regions, with the proteins being excluded from the gel phase lipid.
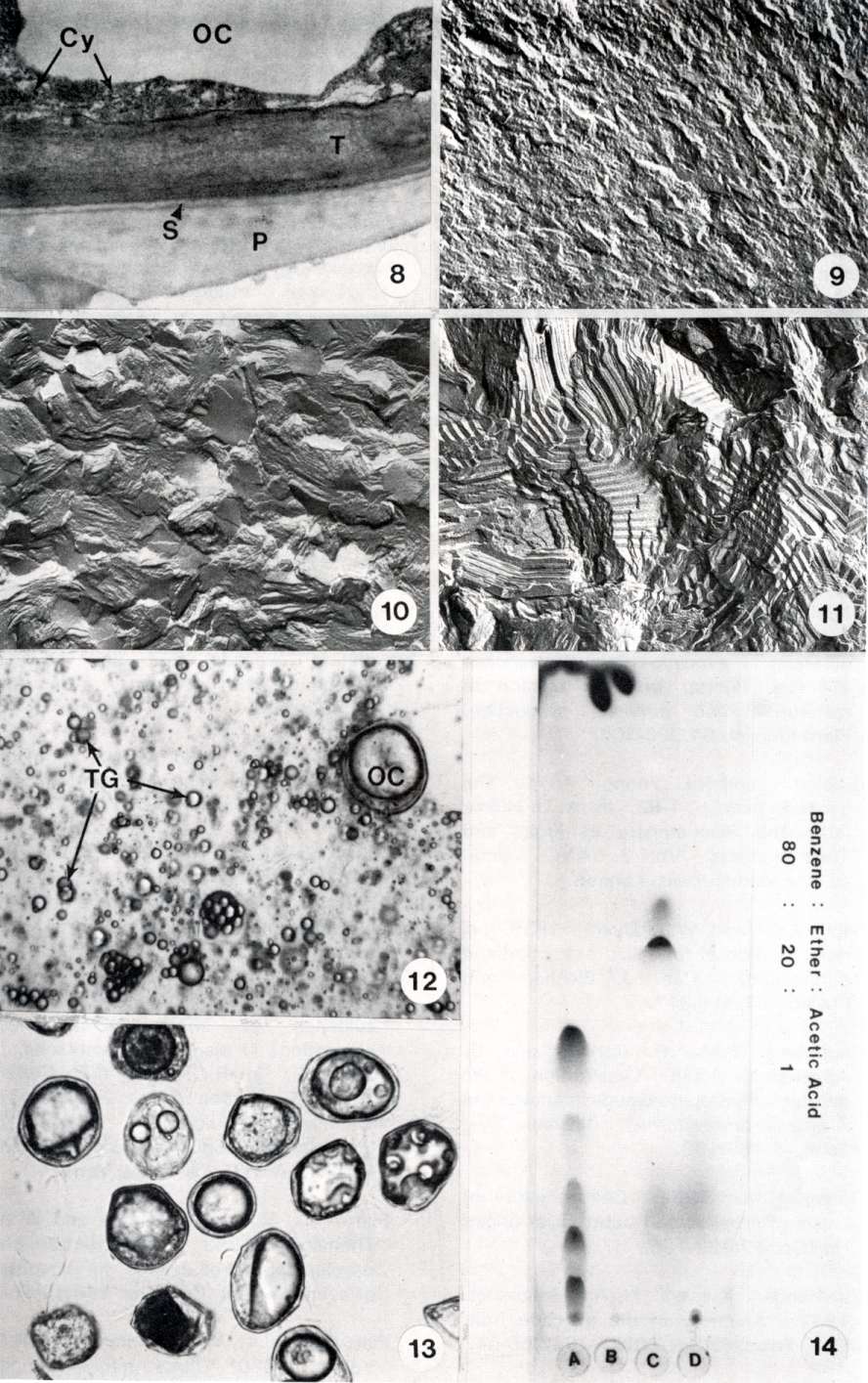
Approximately 2% of the cells of the avocado mesocarp are specialized (idioblast) oil cells (Fig. 7). These cells are filled with lipid, with apparently degraded cytoplasm around the periphery. The cells are surrounded by a complex cell wall composed of a primary wall, a suberized layer, and a tertiary wall (Fig. 8). The oil contained in these cells stains with a different density compared to the lipid in the parenchyma cells (Fig. 7). Additionally, when tissue is freeze fractured, the oil of the oil cells has a different consistency than the oils in the parenchyma cells (Figs. 9 & 10). In freeze fracture of fruit that have been stored at 6C for several days, the oil of the oil cells appears to have crystallized (Fig. 11), while the oils in the parenchyma cells do not change in structure or organization.
During fruit ripening, the suberized oil cell walls, unlike the walls of the parenchyma cells, do not become entirely degraded. These cells are therefore relatively easy to isolate from the rest of the cells. By grinding the tissue in distilled water, the wall-less parenchyma cells burst due to the osmotic shock, while the oil cells maintain their integrity. Thus, the oil cells can be separated from the rest of the cell debris by filtration and centrifugation. Figure 12 shows a light micrograph of the total homogenate of avocado mesocarp, and Fig. 13 of the isolated oil cells.
Thin layer chromatography of the lipid extract of isolated oil cells indicates a complex lipid composition (Fig. 14).
Discussion
Ultrastructural changes in the cell walls of ripening avocado fruit reflect the effect of the high activity of wall hydrolytic enzymes during ripening. The loosening of the cellulose fibrils indicates a loss of the matrix material of the wall, probably due to the increased activity of polygalacturonase (Awad and Young, 1979). The degradation of cellulose, due to cellulase activity, is most evident in samples taken from very soft, post-climacteric fruit, where the cell walls are virtually solubilized, and transparent to the electron beam.
In numerous studies on the effect of stress (eg. senescence and low temperatures) on the functioning of plants, one of the criteria often used for damage is an increased leakiness of the cells (Levitt, 1980). This loss of control of permeability is an indication of damage to the function of the plasmalemma. Freeze fracture electron microscopy is a powerful technique for the study of membrane structure. In the present, and previous (Platt-Aloia and Thomson, 1981) studies, freeze fracture of the plasmalemma of avocados has shown that while there is no indication of damage to the plasmalemma of avocado cells during ripening, there is a significant change in the organization of this membrane in fruit that show injury due to low temperatures. The apparent phase separations of the membrane components imply a change of the phase state of some of the membrane lipids from a fluid to a gel phase (Moeller et al., 1981). Studies using model systems of membrane Iipids have shown that liposomes with a mixture of both fluid and gel phase Iipids exhibit increased permeability when compared to systems composed of fluid phase Iipids only (Haest et al., 1972). Thus it is reasonable to suppose that the membranes of avocados with a mixture of lipid phases would exhibit increased leakiness, and loss of selective permeability properties which are necessary for the proper functioning of the cell. The resulting membrane dysfunctioning would lead to cell death, causing visible chilling injury.
The presence of the idioblastic
oil cells in the mesocarp of avocado fruit is of interest.
Similar oil cells have been reported to occur in the leaves, stems, and roots
of avocados (Armstrong, 1964), and are widespread in other plants (West, 1969).
The ultrastructure of idioblasts reported in Laurus leaves (Maron
and Fahn, 1979) is essentially the same as in
avocados. Ultrastructural studies indicate a unique
composition of the oil, based both on staining characteristics, and on freeze
fracture patterns of the oil at room temperature, and at low, nonfreezing
temperatures. Analyses of the oils contained in other species have shown the
presence of sesquiterpene lactones (Cappelletti et al., 1986), and Scott et
al. (1963) considered these cells in avocado to contain terpenoids. Thin layer chromatography of lipid extracts of
the isolated avocado oil cells shows the presence of several different
components, all of which are different from the bulk, triglyceride, lipid of the fruit. Further analyses will be necessary to
characterize and identify these components. The ease with which these oil cells
can be isolated from ripe avocados is encouraging to the pursuit of
investigation of possible economic value of the oil, as it could be a source of
income from culls and otherwise useless fruit.
Literature Cited
Armstrong,
W.W., Jr. 1964. Distribution of oil cells in Persea.
Masters Thesis.
Awad, M. and R.E. Young. 1979.
Biale, J.B. and R.E. Young. 1971. The avocado pear, p. 1-63. In: A. C. Hulme (ed.) The Biochemistry of
Fruits and Their Products. Vol. 2. A. C. Hulme, ed. Academic Press,
Bligh, E.G. and W.J. Dyer. 1959. A rapid method of total lipid extraction and
purification.
Cappelletti, E.M., R. Caniato and G. Appendino. 1986. Localization of the cytotoxic hydroperoxyeudesmanolides in Artimesia umbelliformis. Biochem. Sys. Ecol. 14:183-190.
Couey, H.M. 1982. Chilling injury of crops of tropical and subtropical origin. HortSci. 17:162-165.
Cummings, K. and
Haest, C.W.M., J. DeGier, G.E. van Es, A.J. Verkleij and L.L.M. Van Deenen. 1972. Fragility of the permeability barrier of Escherichia coli. Biochim. Biophys. Acta 288:43-53.
Levitt,
J. 1980. Responses of plants to environmental stresses, 2nd
edition, Vol I; Chilling, Freezing, and High
Temperature Stresses. Academic Press,
Maron, R. and A. Fahn. 1979. Ultrastructure and development of oil cells in Laurus nobilis L. leaves. Bot. J. Linnean Soc. 78:31-40.
Moeller, C.H., J.B. Mudd and W.W. Thomson. 1981. Lipid phase separations and intramembranous particle movements in the yeast tonoplast. Biochim. Biophys. Acta 643:376-386.
Platt-Aloia, K.A. and W.W. Thomson. 1981. Ultrastructure of the mesocarp of mature avocado fruit and changes associated with ripening. Ann. Bot. 48:451-465.
Platt-Aloia, K.A. and W.W. Thomson. 1982. Freeze fracture of intact plant tissue. Stain Technol. 57:327-334.
Platt-Aloia, K.A. and W.W. Thomson. 1987. Freeze fracture evidence for lateral phase separations in the plasmalemma of chilling injured avocado fruit. Protoplasma 136:71-80.
Platt-Aloia, K.A. 1988. Freeze fracture evidence of stress-induced phase
separations in plant cell membranes, p. 259-292. In: R.C. Aloia,
C.C. Curtain and L.M. Gordon (eds.) Advances in Membrane Fluidity. Vol. 3. Physiological Regulation of Membrane Fluidity. Alan R. Liss,
Platt-Aloia, K.A., J.W. Gross and W.W. Thomson. 1983. Ultrastructure and development of oil cells in the mesocarp of avocado fruit. Bot. Gaz. 144:49-55.
Platt-Aloia, K.A., W.W. Thomson and R.E. Young. 1980. Ultrastructural changes in the walls of ripening avocados: Transmission, scanning, and freeze fracture electron microscopy. Bot. Gaz. 141:366-373.
Scott, P.M., B.C. Bystrom
and E. Bowler. 1963. Persea
Spurr, A.R. 1969. A low viscosity epoxy resin embedding medium for electron microscopy. J. Ultrastruct. Res. 26:31-43.
West, W.C. 1969. Ontogeny of oil cells in the woody Ranales. Bull. Torrey Bot. Club 96:329-344.
Fig. 1. Electron micrograph of a portion of a parenchyma cell of an unripe avocado. The cell wall (W) is a compact array of fibrils and matrix. The endoplasmic reticulum (ER) is in the form of tubes or sheets. M = mitochondria, L = lipid, V = vacuole. X 11,000.
Fig. 2. Electron micrograph of portions of three cells of an avocado at the climacteric peak. The cell walls (W) show a loosening of the fibrils and a loss of electron density. The endoplasmic reticulum (ER) is swollen and vesiculate. X 9,600.
Fig. 3. Electron micrograph of the cell wall (W) of a soft, post- climacteric avocado. The wall has lost almost all of its components and is highly electron transparent. Vesicles in the wall are probably remnants of plasmodesmata. ER = endoplasmic reticulum vesicle. X 14,200.
Fig. 4. Electron micrograph of a freeze fracture replica of the plasmamembrane of a parenchyma cell of an unripe avocado. The integral membrane proteins are evenly distributed in the plane of the membrane. X 60,000.
Fig. 5. Freeze fracture replica of the plasmamembrane of a cell from a ripe, post-climacteric avocado. The intramembrane protein particles are evenly distributed. X 66,000.
Fig. 6. Freeze fracture replica of the plasmamembrane of a chilling-injured avocado. The star designates a region that is depleted of integral proteins indicating the formation of gel phase lipid, from which the proteins have been excluded. X 49,000.
Fig. 7. Electron micrograph of several
cells of the mesocarp of an unripe avocado.
The oil cell (OC) is filled with a light-staining oil. The surrounding
parenchyma cells contain numerous electron dense lipid droplets (L). Around the
periphery of the oil cell is an electron dense cytoplasm, and a specialized
cell wall. X 670.
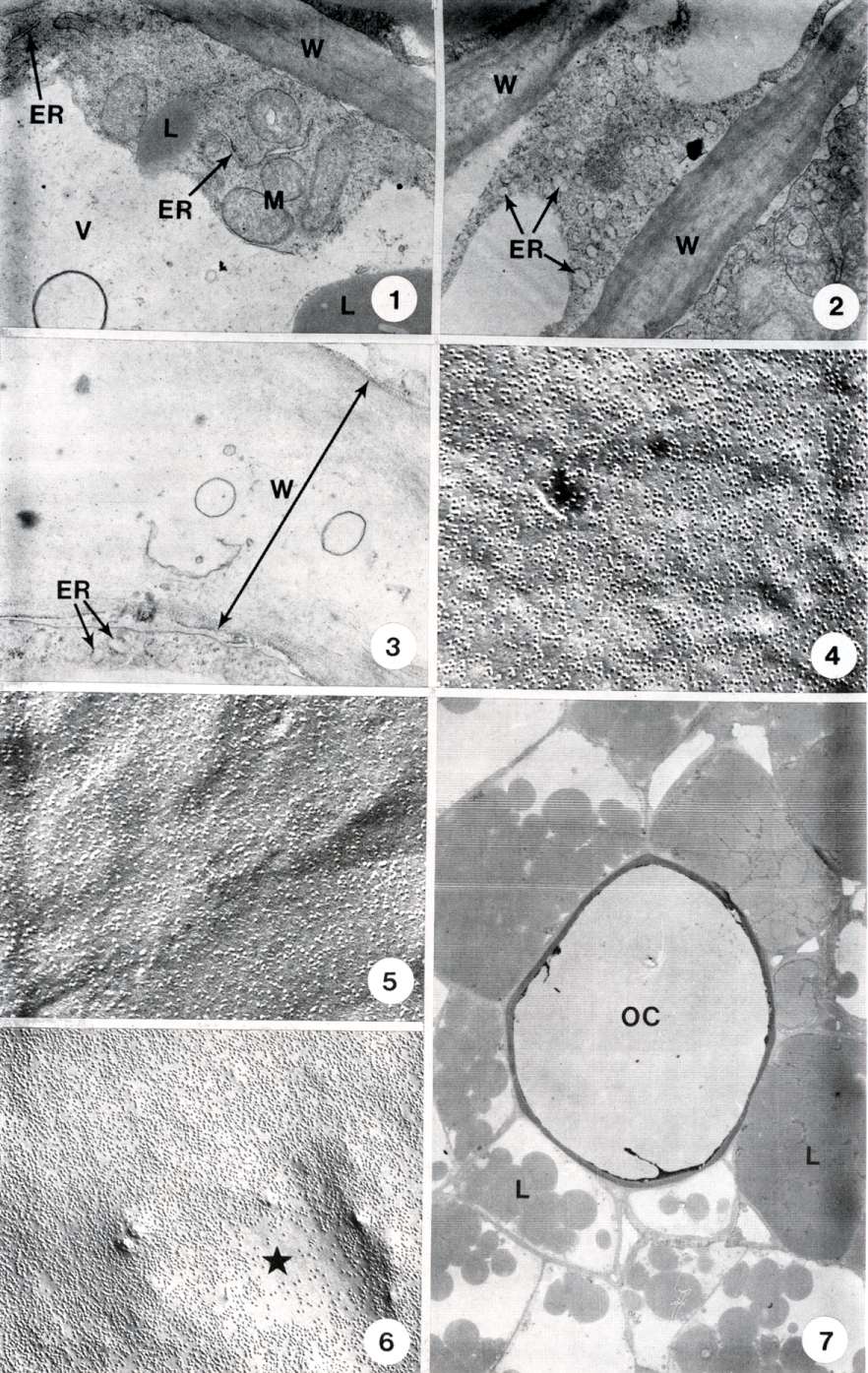
Fig. 8. Electron micrograph of a portion of the specialized cell wall of the oil cell (OC) of an avocado. The wall consists of a primary wall (P), a median suberized layer (S), and a tertiary wall (T). The cytoplasm (Cy) is apparently degenerated. X 12,000.
Fig. 9. Freeze fracture replica of the storage oils of a parenchyma cell of an avocado. The irregular, rugged fracture plane is typical of triglycerides. X 27,200.
Fig. 10. Freeze fracture replica of the oil of an idioblast oil cell frozen from room temperature. The pattern of the fracture plane is different from that shown in figure 9 of the oil in parenchyma cells. X 32,300.
Fig. 11. Replica of the oil of an oil cell that was frozen for freeze fracture after storage at 6C for 2 weeks. The regular pattern is indicative of a crystallization of the oil at this temperature. The oils in the parenchyma cells do not show this temperature effect. X 32,300.
Fig. 1 2. Light micrograph of the homogenate of a ripe avocado. The major component is oil droplets (TG). OC = oil cell. X 170.
Fig. 13. Light micrograph of isolated oil cells. X 170.
Fig. 14. Thin layer chromatogram of oil extracted from
isolated oil cells and lipid standards. A = Oil Cell extract, B =
Triglycerides, C = Diglycerides, D = Phosopholipids.