Proc. of
Parasitoids of the Heart-Shaped (Pyriform)
Scale. Protopulvinaria pyriformis
(Cockerell) (Hemiptera:
Coccidae) on Avocados in
W. J. Du Toit, Marinda S. Schutte, and W. P. Steyn
Citrus and
Subtropical Fruit Research Institute, Private Bag X11208, Nelspruit
1200, Republic of South Africa
Abstract. The susceptibility of the various stages in a field population of the heart-shaped scale Protopulvinaria pyriformis (Cockerell) to parasitoids was investigated at biweekly intervals over a period of one year. Parasitism for the different instars varied, e.g., up to 70% parasitism was recorded in the adult female stage, whereas only 4% was recorded for the second instar. A 7% parasitism of the third instar occurred during the first generation (February) and 20% during the second generation (October). Up to 25% of all stages in the total population, except the first instar of the scale, were parasitized. The parasitoid species, probably Metaphycus galbus Annecke, M. helviolus Compere and M. Stanleyi Compere, played a major role and were responsible for approximately 64% of the total parasitism in this study, while Coccophagus and Tetrastichus spp. accounted for the remainder.
The
heart-shaped or pyriform scale Protopulvinaria
pyriformis (Cockerell)
was discovered in
Materials and Methods
A
biweekly population survey was carried out in a 25-year-old avocado orchard,
cultivar Fuerte, at Woodhouse, Nelspruit,
(30° 50' E and 25° 27' S). During each survey, approximately 15 to 25
scale-infested leaves from a block of 25 data trees were collected. All the
scale on each leaf were examined and separated according to their stage of
development and physical condition, i.e., alive, dead or parasitized.
Parasitized individuals were further divided into larvae, pupae and emerged
adult parasitoids. Every parasitized individual was removed and placed in a
gelatin capsule for adult emergence. Seven morphological stages of the scale
were defined, namely: crawler, first instar, second instar, third instar, A- female, ring stage and A+ female. As a result of the
short development period of certain stages, the development period of the scale
was divided into four stages, namely: the first instar
which includes the crawlers, the second instar, the
third instar which includes mature, unsclerotised, females without eggs (A- stage) and finally
the ring stage plus the mature, sclerotised females
with eggs (A+ stage). The survey, which was carried out on the spring flush,
started in January, 1989, and ended in October, 1989, when the leaves started
to drop.
Results
The
parasitoids, which have not yet been identified to the species level, are
divided into four groups; namely, group A, Metaphycus
spp. A (Encyrtidae);
group B, Metaphycus spp.
B; group C, Coccophagus spp. (Aphelinidae) and Tetrastichus spp. (Eulophidae) and group D,
The First Instar. The survey of the first instar of the second generation heart-shaped scale showed a peak of 30 days from early April to early May (Fig. 1). No parasitism was found in this instar, possibly because the individuals of the first instar are too small to be parasitized by the parasitoids.
The Second Instar. The second instar of the second
generation occurred from the beginning of April to the middle of October (Fig.
2). The first generation ended at the end of March. The second instars of the
first and second generation overlapped in such a way that second instars were
always present.
Parasitism of 3% and less was found in the second instar. Group C parasitoids were dominant during the entire second generation peak and represented 82.8% of the parasitism (Fig. 3), while group A parasitoids represented the other 17.2%. A definite parasitoid peak followed each instar's peak. (Fig. 2).
The Third Instar. The third instar
individuals of the first generation were present from January to the first week
in April, with a peak occurring in the third week of February (Fig. 4). The
second generation's third instar occurred from the
middle of June until November. A gradual accumulation of parasitoids was
noticed from the middle of January and reached a peak of 44% at the beginning
of May (Fig. 4). Initially groups A and C were dominant but were gradually
overshadowed by group B (Fig. 5). Parasitism during the second generation later
in 1989 was very low (3%) and only started increasing from the third week in
September (Fig. 4). During this period, group C was most prominent (up to
85.7%) while group B decreased gradually. The hyperparasitoid
The Mature Female (A +). The
A+ stage extended from the last week in February to the middle of June (Fig. 6)
and was followed by a gradual increase in parasitism to 70% in the middle of
May. High parasitism of 40% to 100% was recorded from middle June to the end of
September, but the A+ females comprised less than 2% of the population during
this period. This parasitism, therefore, had little influence on the scale
population. As far as the relationship between the different parasitoids is
concerned, group A represented 35% to 40% of the parasitism at the beginning of
the A+ stage, but decreased rapidly later (Fig. 7). Group B was initially
responsible for approximately 40% of the parasitism which doubled in early May.
Group C played a minor role, except at the end of the A+ stage in May, where it
represented 50% of the parasitism. The hyperparasitoid
At
the end of the experiment, the scale population averaged approximately
225 individuals/leaf. The excessive excretion of honeydew and sooty
mould which developed on it caused mainly the lower half of the trees to appear
black.
Conclusions
The
complex of the parasitoids of the heart-shaped scale changes in conjunction
with the developmental stage of the host and the season. No parasitoid group
played a dominant role during the entire season. The Metaphycus
groups A and B, which were dominant during late summer and autumn during
the first generation scale, were mostly replaced in winter by Coccophagus and Tetrastichus
group C. This parasitoid complex, which parasitized up to 25.2% of the
total scale population during autumn, did not have the ability to reduce the
host's numbers effectively during this trial.
Thanks are due to Dr M.A. van den Berg for critically
reading the manuscript. This publication forms part of a Ph. D. thesis to be
submitted to the University of the
Literature Cited
Brian, C.K. 1920. The Coccidae
of
De Villiers, E.A. 1981. Hartvormige dopluis op avokado's. H.4. Farming in
De Villiers,
E.A. 1989. Seisoensvoorkoms van verskillende stadia van die hartvormige dopluis
op avokado's. S.A. Avocado Growers'. Assn. Yrbk. 12:58-59.
Du Toit, W.J. and E.A. De Villiers. 1988. Die hartvormige dopluis, Protopulvinaria pyriformis (Cockerell) op avokado's. S.A. Avocado Growers'. Assn. Yrbk. 11:79-80.
Prinsloo, G.L. 1984. An illustrated guide to the parasitic
wasps associated with citrus pests in the
Robertson, C.M. and E.A. De Villiers.
1986. Parasites of avocado pest bite the dust. Information Bulletin no. 168,
CSFRI,
Wysoki, M. 1987. A bibliography of the pyriform scale Protopulvinaria
pyriformis (Cockerell),
1894 (Homoptera: Coccidae),
up to 1986. Phytoparasitica 15:73-77.
|
|
|
|
|
|
|
|
|
|
|
|
|
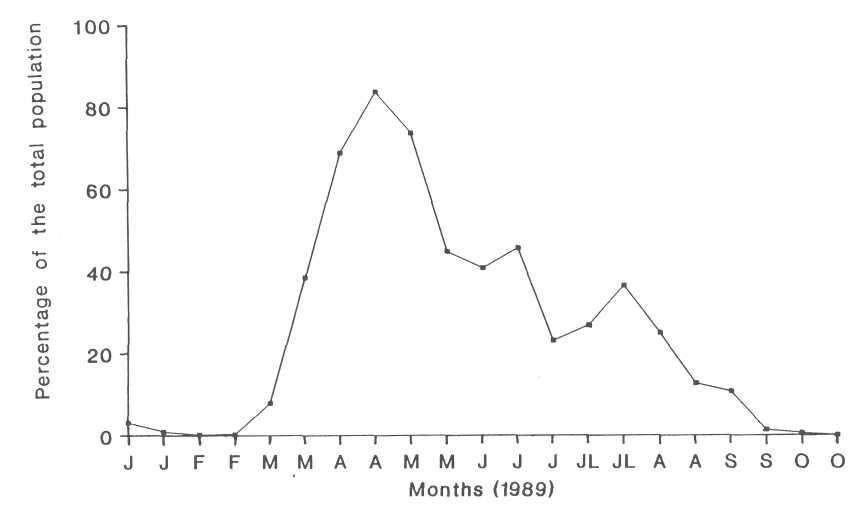
Fig. 1. The occurrence of the first instar nymphs of the heart-shaped scale.
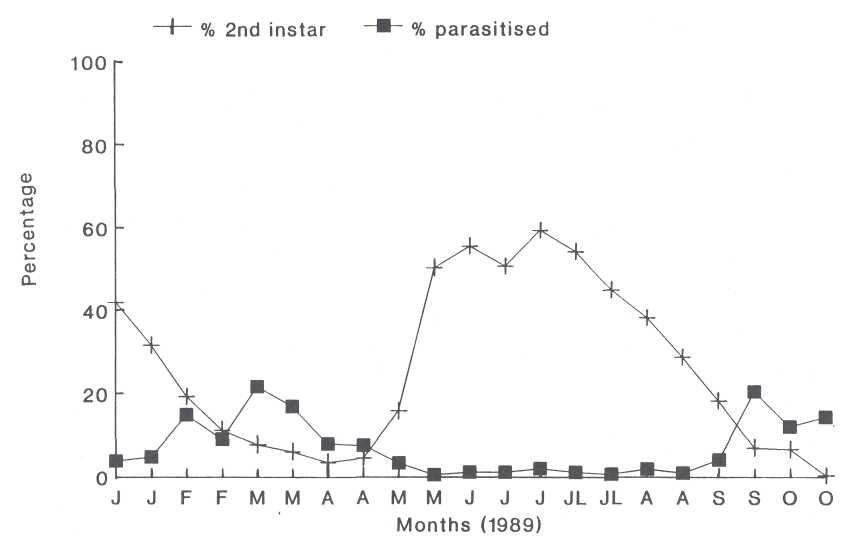
Fig. 2. The occurrence of the second instar nymphs of the heart-shaped scale as a percentage of the total population and the percentage parasitized.
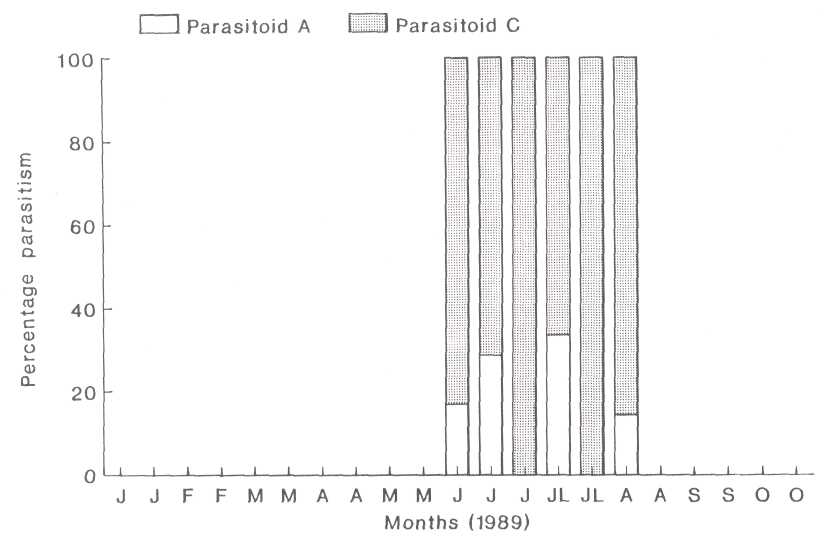
Fig. 3. The relationship between the parasitoids which were found in the second instar nymphs of the heart-shaped scale.
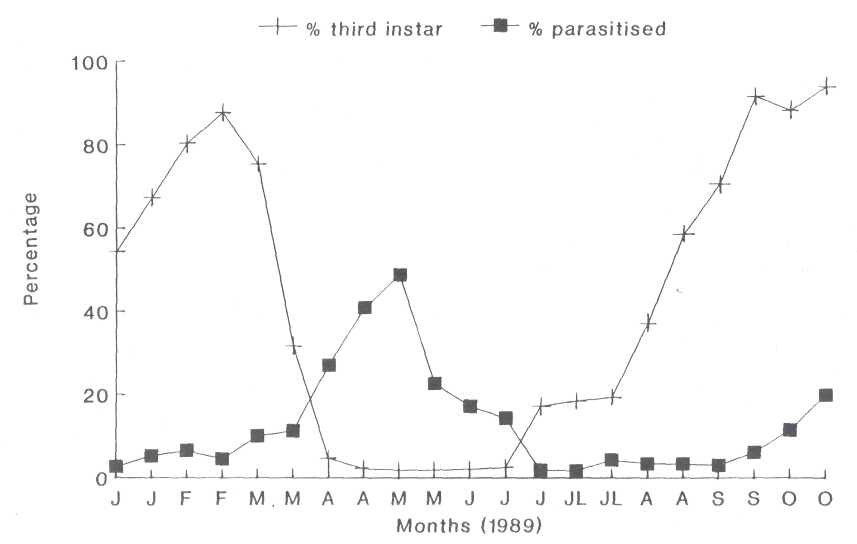
Fig. 4. The occurrence of the third instar nymphs of the heart-shaped scale as a percentage of the total population and the percentage parasitized.
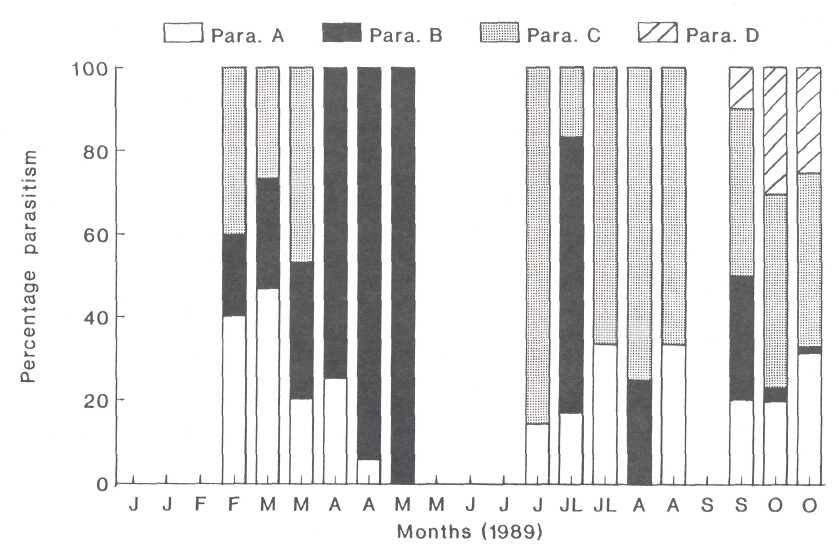
Fig. 5. The relationship between the parasitoids which were found in the third instar nymphs of the heart-shaped scale.
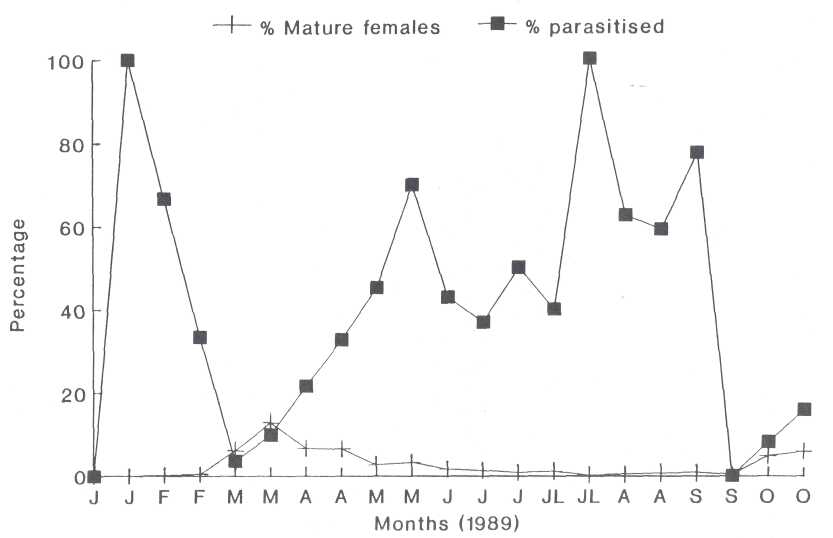
Fig. 6. The occurrence of the mature females (A +) as a percentage of the total population and the percentage parasitized.
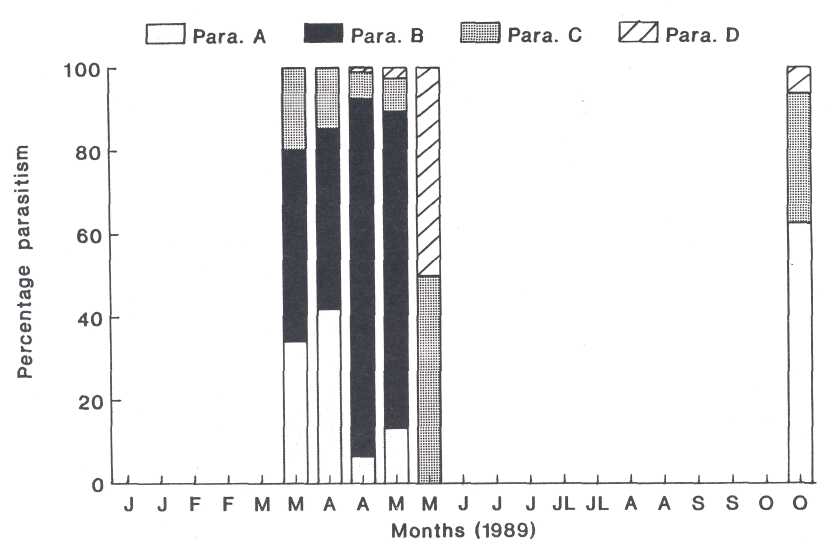
Fig. 7. The relationship between the parasitoids which were found in the mature females of the heart-shaped scale.
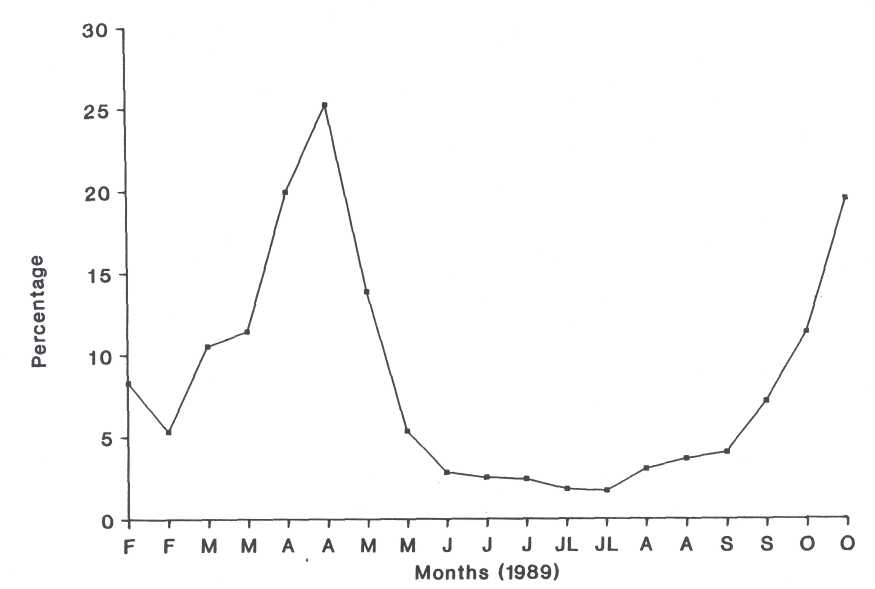
Fig. 8. Percentage parasitism of the total population during the season with exception of the first instar (n = 2000).